Published research now highlights the prognostic and predictive value of blood-based circulating tumor DNA (ctDNA) testing for the management of stage II-IV melanoma and shows how personalized tumor-informed molecular residual disease (MRD) test monitoring with ctDNA can inform treatment decisions, monitor for recurrence, and assess response to treatment.1,10

What is circulating tumor DNA?
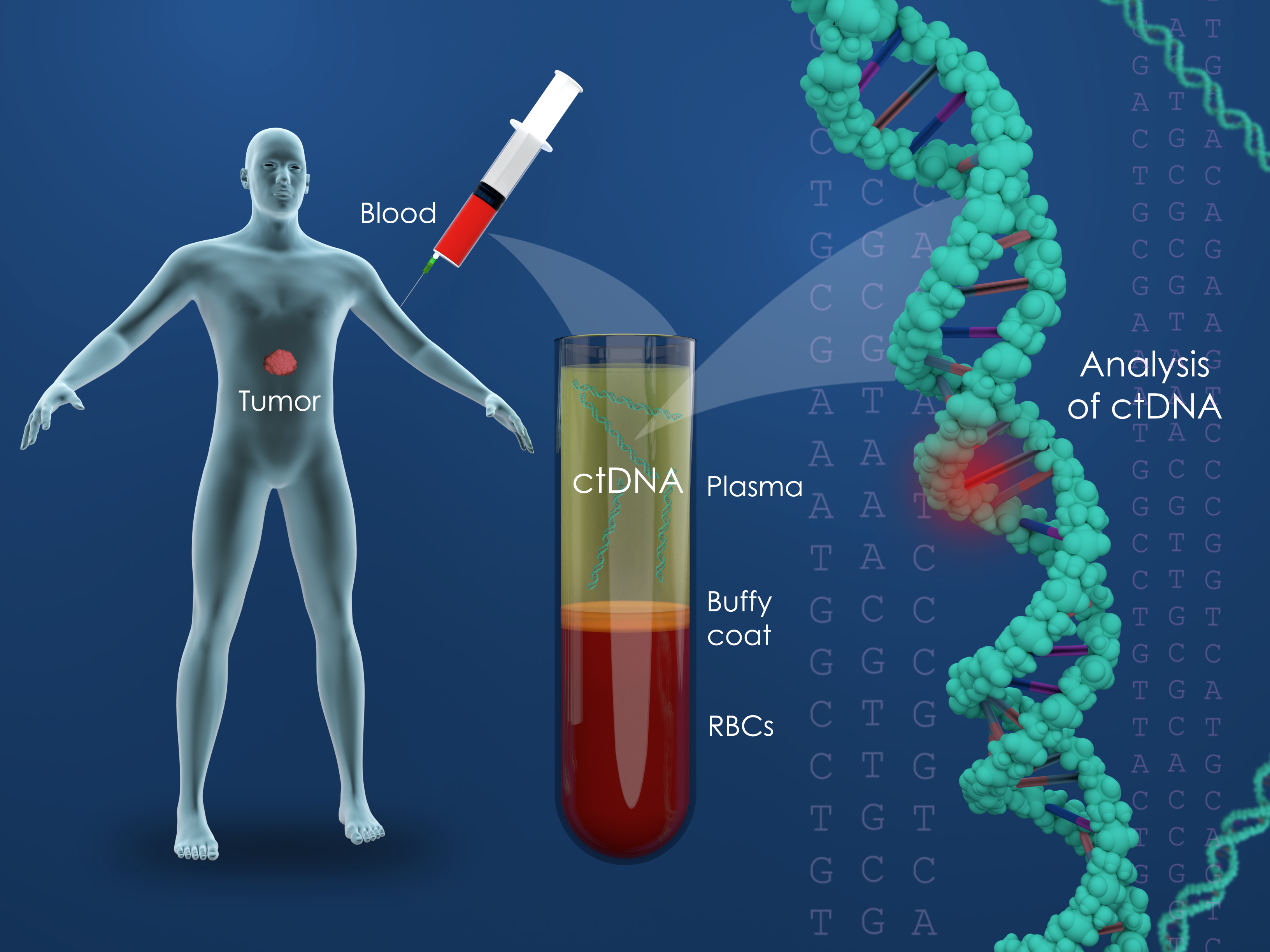
Circulating tumor DNA (ctDNA) is small fragments of DNA, which originate from cancer cells and are present in the bloodstream or other body fluids. Cancer is caused by genetic mutations, and these mutations can be detected by measuring the ctDNA, in the blood. There are two general types of ctDNA tests; “informed” and “uninformed.” Because each cancer is as unique to a person as their own fingerprint gene sequencing can be used to identify the markers of a patient’s own cancer. These tumor informed ctDNA assays are therefore a personalized cancer-monitoring test that is built from an individual’s actual cancer and is unique for that person. This stands in contract to “uninformed’ tests which use panels of known genetic and epigenetic cancer markers and tend the be less specific.
In the current reports prospectively collected “blood” samples from patients with stages II-IV melanoma using the “tumor informed” Signatera assay were analyzed. Key study takeaways include:
Stage II-III Melanoma: In individuals with surgically treated stage III melanoma a positive Signatera test post-surgery was associated with significantly shorter survival and identified individuals most likely to benefit from adjuvant therapy. Signatera detected recurrence with an average lead time of 3 months over standard imaging tests. All patients who developed detectable ctDNA during surveillance experienced a melanoma recurrence.2,9
Stage III/IV Melanoma Treated with Immunotherapy: An increase in ctDNA levels 3-11 weeks after starting immune checkpoint inhibitor therapy was associated with more rapid disease progression and reduced survival. All patients with increasing ctDNA experienced disease progression, while all patients with decreasing ctDNA achieved complete or partial response.
Stage III/IV Melanoma Undergoing Surveillance after Completion of Immunotherapy: Although a small sample, 7 of 7 individuals who were ctDNA-negative during post treatment surveillance remained free of melanoma through their most recent follow up and all ctDNA-positive patients experienced disease progression.
The use of immune checkpoint inhibitors has led to significant improvements in overall survival rates for patients with advanced melanoma.2-7Response to treatment however can be difficult and standard imaging techniques with PET/CT expose patients to radiation, are costly, and detect recurrences several months later than ctDNA. Moreover side effects from checkpoint inhibitors can be significant.
Surveillance for MRD with ctDNA testing can lead to early identification of recurrence or complete response resulting in shorter immunotherapy treatment duration and avoidance of potential side-effects.
- Help avoid the high cost of ineffective therapy
- Avoid side effects of ineffective therapy
The key question being evaluated in clinical trial is “Does ctDNA detection mediated change in therapy improve outcomes?”

Patient Submitted Questions
What is difference between ctDNA tests for early cancer detection vs MRD?
Early detection tests are similar but not identical to MRD tests. Early detection tests are typically optimized to detect the early presence of an unknown malignancy. MRD tests are designed to detect the presence of a cancer that was previously known to be present but removed. As a general rule, an early detection test is broad (can detect multiple cancers) but is not as sensitive for 1 specific cancer. MRD tests are more sensitive for 1 specific cancer, but are not designed to detect other cancers.
What is difference between tumor-naive vs. tumor-informed MRD assays. How much do we know about ctDNA as a forecasting tool?
Tumor naïve tests do not require genomic sequencing of tumor tissue. These tests rely on a standard mutation and methylation panel that is common to the cancer under study. Tumor informed tests require sequencing the patient’s tumor, and then building a custom panel to detect those tumor mutations in blood. We have no head to head studies comparing the two different approaches.
I have had nine negative Signatera tests and now a positive. What are the chances I really do have a recurrence versus a false positive?
False positives are extremely rare, but there have been reports of transient positive results that spontaneously clear. If there is any doubt, I recommend rechecking the test 4-8 weeks after the positive result to confirm. If the ctDNA result remains positive it confirms the result. Liver MRI is a great test to consider if the CT scan is clear.
How reliable is ctDNA testing during chemotherapy treatment? Should the test be performed while on chemotherapy?
ctDNA testing is less likely to be positive during chemotherapy treatment. Despite that, it can still be useful during treatment. Reduction in ctDNA is associated with favorable response to treatment and better prognosis.
Why do these tests sometimes miss cancer in the lungs/liver?
These tests rarely miss disease in the liver. MRD tests tend to miss disease in the lungs, because those metastases secrete less circulating tumor DNA into the bloodstream and tend to be slower growing overall (less cell turnover to release DNA)
Can CTDNA go up as part of pseudoprogression on immunotherapy?
No… pseudoprogression is associated with lower circulating tumor DNA levels, but worsening disease on imaging.
References:
- https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.34716
- Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat. Cancer. 2020;1(9):873-881.
- Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with BRAF(V600) mutated metastatic melanoma: an open-label, multicentre, safety study. Lancet Oncol. 2014;15(4):436-444.
- Chapman PB, Robert C, Larkin J, et al. Vemurafenib in patients with BRAFV600 mutation-positive metastatic melanoma: final overall survival results of the randomized BRIM-3 study. Ann Oncol official J Eur Soc Med Oncol. 2017;28(10):2581-2587.
- Robert C, Grob JJ, Stroyakovskiy D, et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N Engl J Med. 2019;381(7):626-636.
- Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label,multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239-1251.
- Wolchok Jdc-S V, Gonzalez R, Grob J-J, et al. CheckMate 067: 6.5-year outcomes in patients (pts) with advanced melanoma. J Clin Oncol. 2021;39(15_suppl):9506.
- Swetter SM, Thompson JA, Albertini MR, et al. NCCN Guidelines® insights: melanoma: cutaneous, version 2.2021. J Natl Compr Canc Netw. 2021;19(4):364‐376.
- Feasibility of personalized circulating tumor DNA detection in stage II and III melanoma. Brunsgaard et al.
-
https://acsjournals.onlinelibrary.wiley.com/doi/full/10.1002/cncr.34716